Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00081
|
|||||
| Drug Name |
Ergotidine
|
|||||
| Synonyms |
1H-Imidazole-4-ethanamine; 1H-Imidazole-5-ethanamine; 2-(1H-Imidazol-4-yl)ethanamine; 2-(1H-imidazol-4-yl)ethan-1-amine; 2-(1H-imidazol-5-yl)ethanamine; 2-(3H-Imidazol-4-yl)-ethylamine; 2-(4-Imidazolyl)ethylamine; 2-Imidazol-4-ylethylamine; 2-[4-Imidazolyl]ethylamine; 4-(2-Aminoethyl)-1H-imidazole; 4-Imidazoleethylamine; 5-Imidazoleethylamine; ALBB-005968; Beta-Aminoethylglyoxaline; Beta-Aminoethylimidazole; Beta-Imidazolyl-4-ethylamine; Beta-aminothethylglyoxaline; Eramin; Ergamine; F411C768-A159-4FC0-A195-291A08BB03AA; Free histamine; H7125_SIGMA; Histamine; Histamine (DCF); Histamine Base; Histamine [USAN]; Histamine, Free Base; Histaminum; Histaminum (TN); Imidazole-4-ethylamine; Istamina; Istamina [Italian]; L-histamine; Theramine; ZERO/004089; [3H]histamine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Migraine [ICD11: 8A80] | Approved | [1] | |||
| Therapeutic Class |
Antiallergic Agents
|
|||||
| Structure |
|
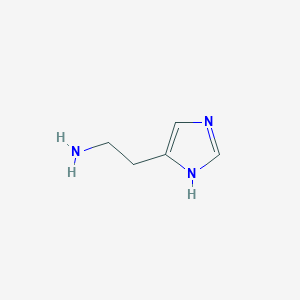 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C5H9N3
|
|||||
| Canonical SMILES |
C1=C(NC=N1)CCN
|
|||||
| InChI |
InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8)
|
|||||
| InChIKey |
NTYJJOPFIAHURM-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 75614-87-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 111.15 | Topological Polar Surface Area | 54.7 | ||
| Heavy Atom Count | 8 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
-0.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10524836
, 11108702
, 11111275
, 11120367
, 11120855
, 11121343
, 11121734
, 11122214
, 11335483
, 11360722
, 11362871
, 11363739
, 11365433
, 11366301
, 11367995
, 11368863
, 11370953
, 11370954
, 11371444
, 11373596
, 11374718
, 11376157
, 11377025
, 11461694
, 11484465
, 11488481
, 11490268
, 11492775
, 11494659
, 11537616
, 15146444
, 16080673
, 3156918
, 3221723
, 3678
, 3727072
, 5065169
, 5186157
, 609692
, 6436474
, 7735853
, 7888228
, 7979989
, 8139979
, 8143824
, 8150782
, 822767
, 824161
, 853773
, 91789
|
|||||
| ChEBI ID |
ChEBI:18295
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | ENT4 | Transporter Info | Equilibrative nucleoside transporter 4 | Substrate | [2] | |
| OCT-2 | Transporter Info | Organic cation transporter 2 | Substrate | [3] | ||
| OCT-3 | Transporter Info | Organic cation transporter 3 | Substrate | [4] | ||
| VMAT1 | Transporter Info | Vesicular amine transporter 1 | Substrate | [5] | ||
| VMAT2 | Transporter Info | Vesicular amine transporter 2 | Substrate | [6] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OCT-2 | Transporter Info | Km = 940 microM | Human embryonic kidney cells (HEK293)-OCT2 | [4] | |
| OCT-2 | Transporter Info | Km = 1300 microM | Oocytes-OCT2 | [7] | ||
| OCT-3 | Transporter Info | Km = 180 microM | Human embryonic kidney cells (HEK293)-OCT3 | [8] | ||
| OCT-3 | Transporter Info | Km = 220 microM | Human embryonic kidney cells (HEK293)-OCT3 | [4] | ||
| References | ||||||
| 1 | Ergotidine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Histamine uptake mediated by plasma membrane monoamine transporter and organic cation transporters in rat mast cell lines. Eur J Pharmacol. 2019 Apr 15;849:75-83. | |||||
| 3 | The organic cation transporters (OCT1, OCT2, EMT) and the plasma membrane monoamine transporter (PMAT) show differential distribution and cyclic expression pattern in human endometrium and early pregnancy decidua. Mol Reprod Dev. 2007 Oct;74(10):1303-11. | |||||
| 4 | Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006 Jun;50(8):941-52. | |||||
| 5 | Gastrin-producing endocrine cells: a novel source of histamine in the rat stomach. Endocrinology. 1998 Oct;139(10):4404-15. | |||||
| 6 | The vesicular monoamine transporter 2: an underexplored pharmacological target. Neurochem Int. 2014 Jul;73:89-97. | |||||
| 7 | Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol. 1998 Aug;54(2):342-52. | |||||
| 8 | Selective substrates for non-neuronal monoamine transporters. Mol Pharmacol. 1999 Jul;56(1):1-10. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
