Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00116
|
|||||
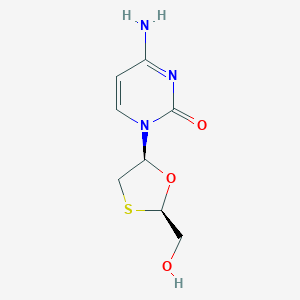
| Drug Name |
Lamivudine
|
|||||
| Synonyms |
(+/-)-(Cis)-1-[2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (+/-)-3TC; (+/-)-BCH-189; (+/-)-SddC; (-)-(2'R,5'S)-1-[2'-Hydroxymethyl-5'-(1,3-oxathiolanyl)]cytosine; (-)-1-((2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine; (-)-1-[(2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (-)-2'-Deoxy-3'-thiacytidine; (-)-BCH 189; (-)-BCH-189; (-)-NGPB-21; (-)-SddC; (-)-beta-L-2',3'-Dideoxy-3'-thiacytidine; (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one; 2',3' Dideoxy 3' thiacytidine; 2',3'-Dideoxy-3'-thiacytidine; 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (+/-)-(Cis); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Galanthus Nivalis Agglutinin (GNA); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Hippeastrum hybrid agglutinin(HHA); 3'-Thia-2',3'-dideoxycytidine; 3TC; 3TC & GNA; 3TC & SST; 3TC (AIDS INITIATIVE) (AIDS INITIATIVE); 3TC and NV-01; 3TC, Zeffix, Heptovir, Epivir, Epivir-HBV, Lamivudine; 4-Amino-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1H)-pyrimidinone; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one; BCH 189; BCH-189; BCH-790; BCH189; Beta-L-(-)-2',3'-dideoxy-3'-thiacytidine & Sho-Saiko-To; Beta-L-2',3'-Dideoxy-3'-thiacytidine; Beta-L-3'-Thia-2',3'-dideoxycytidine; DRG-0126; DTHC; Epivir; Epivir (TN); Epivir(TM); Epivir-HBV; Epivir-HBV (TN); GG-714; GR-109714X; GR109714X; HHA & 3TC; HHA & Lamivudine; Hepitec; Heptivir; Heptodin; Heptovir; Heptovir (TN); LMV; Lamivir; Lamivudine & GNA; Lamivudine (JAN/USP/INN); Lamivudine [USAN:BAN:INN]; Lamivudine [USAN:INN:BAN]; Lamivudine, (2S-cis)-Isomer; Zeffix; Zeffix (TN); Zefix
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic hepatitis B infection [ICD11: 1E51.0] | Approved | [1] | |||
| Human immunodeficiency virus infection [ICD11: 1C62.Z] | Approved | [1] | ||||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H11N3O3S
|
|||||
| Canonical SMILES |
C1C(OC(S1)CO)N2C=CC(=NC2=O)N
|
|||||
| InChI |
InChI=1S/C8H11N3O3S/c9-5-1-2-11(8(13)10-5)6-4-15-7(3-12)14-6/h1-2,6-7,12H,3-4H2,(H2,9,10,13)/t6-,7+/m0/s1
|
|||||
| InChIKey |
JTEGQNOMFQHVDC-NKWVEPMBSA-N
|
|||||
| CAS Number |
CAS 134678-17-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 229.26 | Topological Polar Surface Area | 113 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
-0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103195019
, 103977094
, 104170203
, 104253324
, 104321740
, 11528367
, 117588081
, 118048664
, 12014700
, 12016245
, 121362453
, 124658974
, 124757443
, 124892101
, 125164247
, 126584422
, 126592947
, 126625491
, 126656696
, 126665392
, 127310181
, 127310182
, 14798125
, 15121620
, 24277302
, 26719826
, 29215254
, 3727051
, 3727058
, 43118163
, 46386600
, 46507855
, 49681736
, 50140269
, 57314135
, 596236
, 601313
, 643736
, 7847419
, 7979719
, 81093205
, 811475
, 8187080
, 85279382
, 87560180
, 92308311
, 92712464
, 92729822
, 9277
, 93166191
|
|||||
| ChEBI ID |
ChEBI:63577
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [3] | ||
| OCT-2 | Transporter Info | Organic cation transporter 2 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OCT-2 | Transporter Info | Km = 46.3 microM | Oocytes-OCT2 | [4] | |
| References | ||||||
| 1 | Salicylic acid was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | The effect of ABCG2 V12M, Q141K and Q126X, known functional variants in vitro, on the disposition of lamivudine. Br J Clin Pharmacol. 2007 Nov;64(5):645-54. | |||||
| 3 | Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos. 2008 Aug;36(8):1616-23. | |||||
| 4 | Genetic variants of organic cation transporter 1 (OCT1) and OCT2 significantly reduce lamivudine uptake. Biopharm Drug Dispos. 2012 Apr;33(3):170-8. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
