Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00225
|
|||||
| Drug Name |
Quercetin
|
|||||
| Synonyms |
2-(3,4-Dihydroxy-phenyl)-3,5,7-trihydroxy-chromen-4-one; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one dihydrate; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one; 3',4',5,7-Tetrahydroxyflavan-3-ol; 3',4',5,7-tetrahydroxyflavon-3-ol; 3,3',4',5,7-Pentahydroxyflavone; 3,3',4',5,7-Pentahydroxyflavone dihydrate; 3,3',4,5,7-Pentahydroxyflavone; 3,5,7,3',4'-Pentahydroxyflavone; 3,5,7-Trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-on; 3,5,7-trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-one; C.I . natural yellow 10; C.I. 75670; C.I. Natural Yellow 10; C.I. Natural red 1; C.I. Natural yellow 10 & 13; CI Natural Yellow 10; CU-01000012502-3; Cyanidelonon 1522; Flavin meletin; KSC-10-126; KSC-23-76; KUC104418N; KUC107684N; Kvercetin; Kvercetin [Czech]; LIM-5662; LNS-5662; Meletin; MixCom3_000183; Natural Yellow 10; P0042; Q 0125; QUE; Quercetin content; Quercetin dihydrate; Quercetine; Quercetol; Quercitin; Quertin; Quertine; Sophoretin; T-Gelb bzw. grun 1; TNP00070; TNP00089; Xanthaurine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Coronary artery disease [ICD11: BA6Z] | Phase 3 | [1] | |||
| Obesity [ICD11: 5B81] | Phase 3 | [1] | ||||
| Therapeutic Class |
Hypoglycemic Agents
|
|||||
| Structure |
|
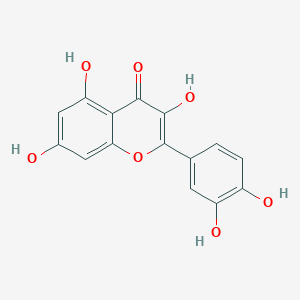 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C15H10O7
|
|||||
| Canonical SMILES |
C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O
|
|||||
| InChI |
InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H
|
|||||
| InChIKey |
REFJWTPEDVJJIY-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 117-39-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 302.23 | Topological Polar Surface Area | 127 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
1.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10318962
, 10318963
, 10589526
, 11108251
, 11111704
, 11111705
, 11111706
, 11113933
, 11120249
, 11120737
, 11121225
, 11121719
, 11122199
, 11335660
, 11360899
, 11362822
, 11362998
, 11365384
, 11365560
, 11367946
, 11368122
, 11370875
, 11370876
, 11371563
, 11373547
, 11374432
, 11376108
, 11376284
, 11387263
, 11401319
, 11446388
, 11455076
, 11455328
, 11461871
, 11466535
, 3157247
, 3679
, 5183365
, 596331
, 74741
, 7636346
, 7884117
, 7890165
, 8137945
, 8145679
, 8149624
, 820896
, 821326
, 841197
, 8616230
|
|||||
| ChEBI ID |
ChEBI:16243
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| FLVCR1 | Transporter Info | Feline leukemia virus subgroup C receptor-related protein 1 | Substrate | [3] | ||
| GLUT1 | Transporter Info | Glucose transporter type 1 | Substrate | [4] | ||
| MCT2 | Transporter Info | Monocarboxylate transporter 2 | Substrate | [5] | ||
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [6] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [7] | ||
| References | ||||||
| 1 | ClinicalTrials.gov (NCT03943459) Sirtuin-1 and Advanced Glycation End-products in Postmenopausal Women With Coronary Disease | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 3 | The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Res. 2016 Jan 4;44(D1):D372-9. (ID: 2.A.1.28.1) | |||||
| 4 | Oral Bioavailability and Disposition of Phytochemicals | |||||
| 5 | Monocarboxylate Transporters in Drug Disposition: Role in the Toxicokinetics and Toxicodynamics of the Drug of Abuse GHB. | |||||
| 6 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
| 7 | Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med. 2004 Mar 1;36(5):592-604. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
