Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00316
|
|||||
| Drug Name |
Oxaliplatin
|
|||||
| Synonyms |
ACT 078; Oxaloplatine; Oxaloplatino; Oxalitin; NSC 271670; Oxalato(1,2-diaminocyclohexane)platinum(II); Platinum (II), (cyclohexane-1,2-diammine)oxalato-; CCRIS 9143; Platinum, (1,2-cyclohexanediamine-N,N')(ethanedioato(2-)-O,O')-, (SP-4-2-(trans))-; Platinum, (1,2-cyclohexanediamine-kappaN,kappaN')(ethanedioato(2-)-kappaO1,kappaO2)-, (SP-4-2-(trans))-; Oxaliplatin [USAN:USP:INN:BAN]; CS-0992; HY-17371; NCI60_002138
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Colorectal cancer [ICD11: 2B91] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
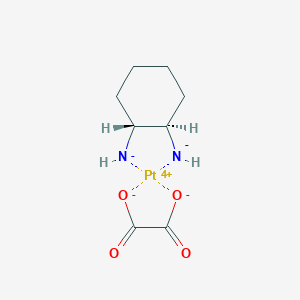 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H12N2O4Pt
|
|||||
| Canonical SMILES |
C1CCC(C(C1)[NH-])[NH-].C(=O)(C(=O)[O-])[O-].[Pt+4]
|
|||||
| InChI |
InChI=1S/C6H12N2.C2H2O4.Pt/c7-5-3-1-2-4-6(5)8;3-1(4)2(5)6;/h5-8H,1-4H2;(H,3,4)(H,5,6);/q-2;;+4/p-2/t5-,6-;;/m1../s1
|
|||||
| InChIKey |
DWAFYCQODLXJNR-BNTLRKBRSA-L
|
|||||
| CAS Number |
CAS 61825-94-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 395.28 | Topological Polar Surface Area | 82.3 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| PubChem CID | ||||||
| PubChem SID |
125309438
, 127301221
, 127301222
, 127301223
, 127301224
, 127301225
, 127301226
, 127301227
, 127301228
, 127301229
, 127301230
, 127301231
, 127301232
, 127301233
, 127301234
, 127301235
, 127301236
, 127301237
, 127301238
, 127301239
, 127301240
, 127301241
, 127301242
, 127301243
, 127301244
, 127301245
, 127301246
, 135684119
, 137001870
, 14854529
, 14903423
, 152059544
, 162036207
, 164196639
, 174477519
, 226396770
, 24173438
, 251885690
, 252156659
, 252316548
, 45633994
, 57373686
, 7848852
, 78790585
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| CTR1 | Transporter Info | High affinity copper uptake protein 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [5] | ||
| OCT-2 | Transporter Info | Organic cation transporter 2 | Substrate | [6] | ||
| References | ||||||
| 1 | Oxaliplatin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Effect of ABCG2 on cytotoxicity of platinum drugs: interference of EGFP. Toxicol In Vitro. 2008 Dec;22(8):1846-52. | |||||
| 3 | Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol. 2005 Jan;53(1):13-23. | |||||
| 4 | Multidrug Resistance-Associated Protein 2 (MRP2) Mediated Transport of Oxaliplatin-Derived Platinum in Membrane Vesicles. PLoS One. 2015 Jul 1;10(7):e0130727. | |||||
| 5 | Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006 Sep 1;66(17):8847-57. | |||||
| 6 | Relevance of copper transporter 1 and organic cation transporters 1-3 for oxaliplatin uptake and drug resistance in colorectal cancer cells. Metallomics. 2018 Mar 1;10(3):414-425. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
